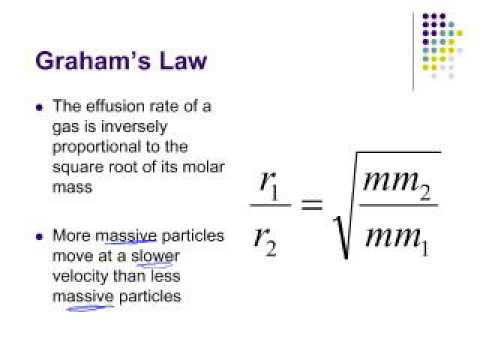
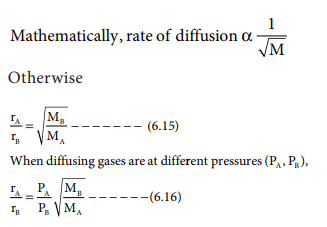
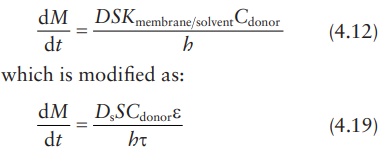
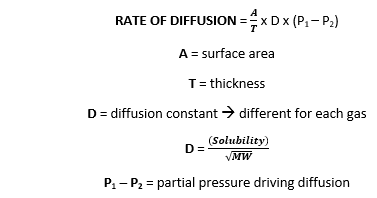
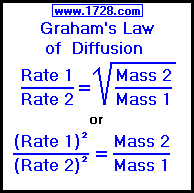
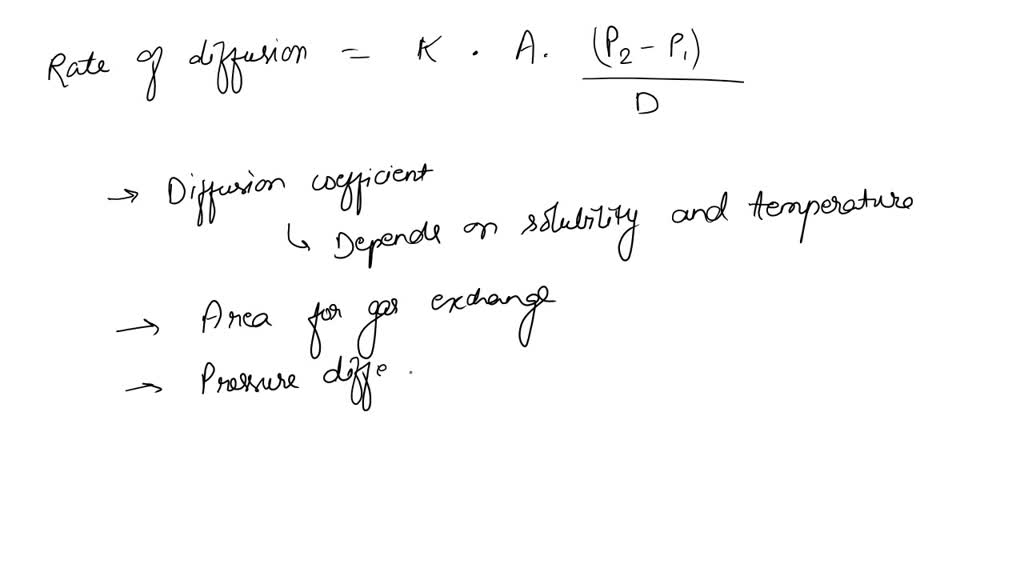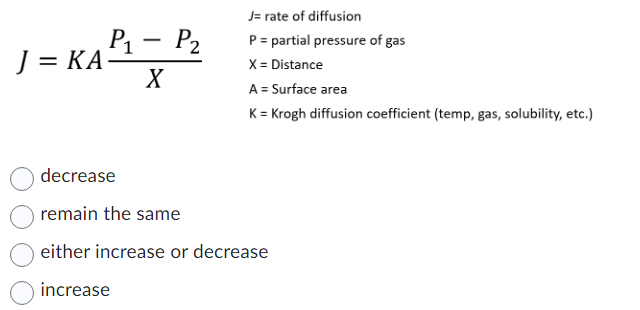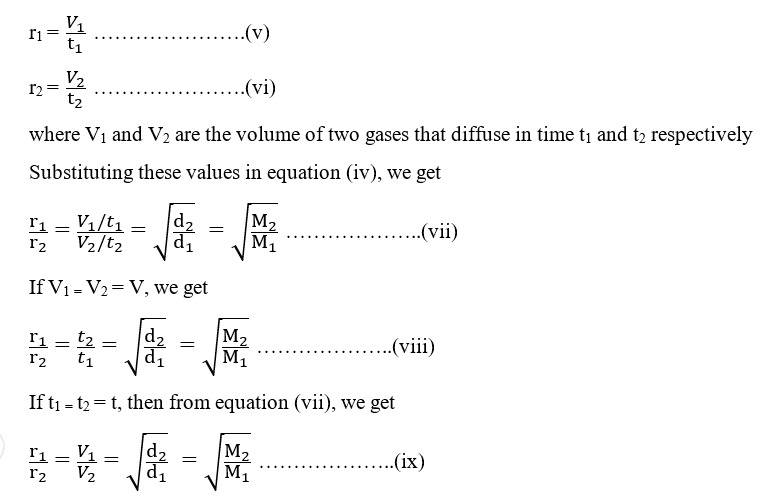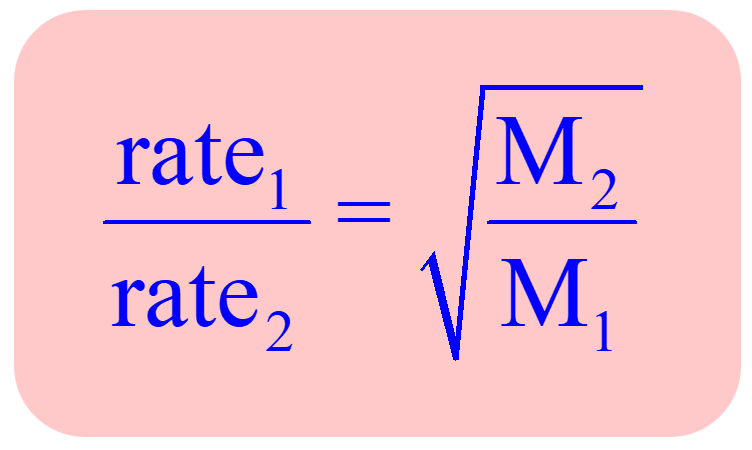The relative rate of effusion of ch4 to so2 through the container containing ch4 and so2 in 3:2mass ratio

Graham's Law of Diffusion Graham's Law KE = ½mv 2 Speed of diffusion/ effusion –Kinetic energy is determined by the temperature of the gas. –At the same. - ppt download