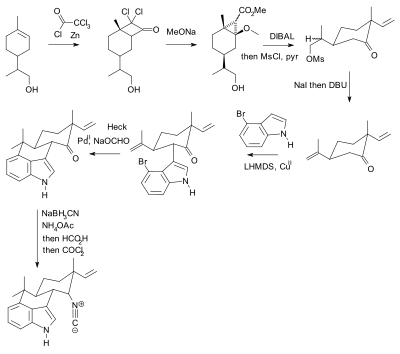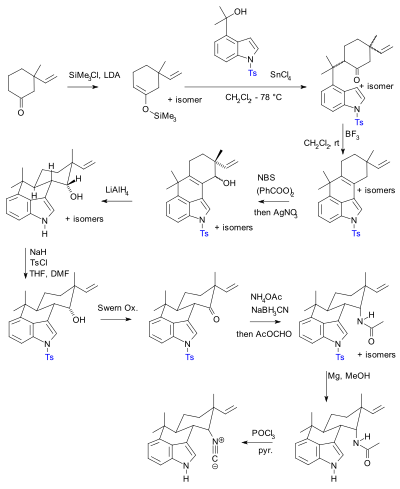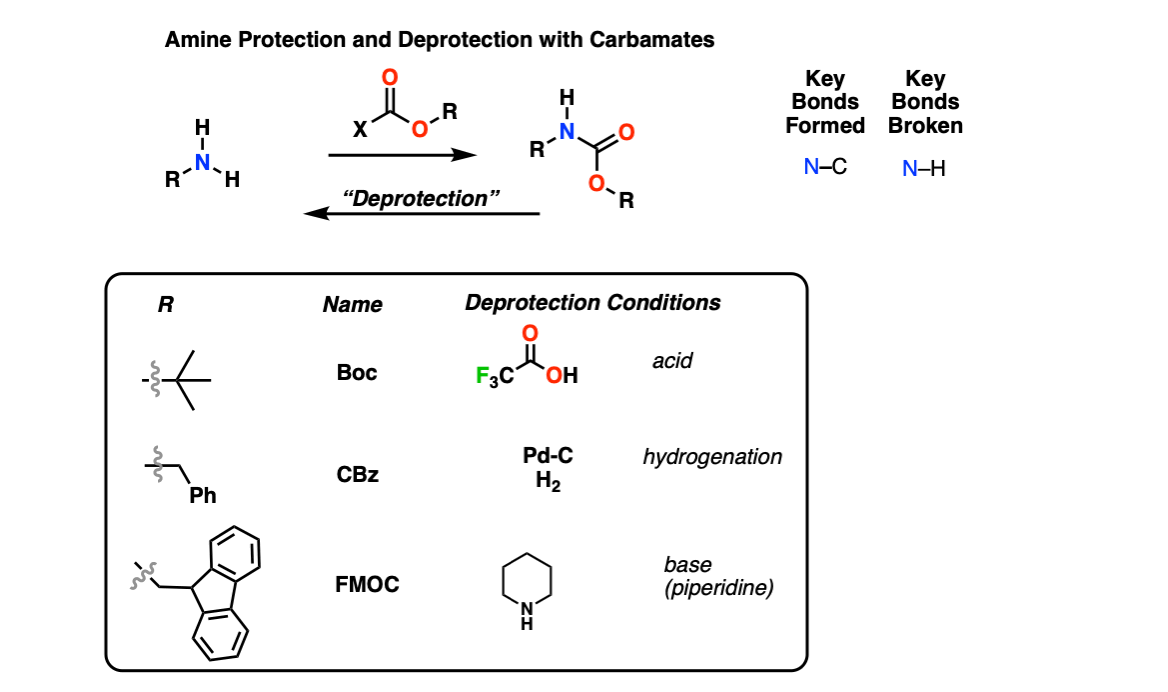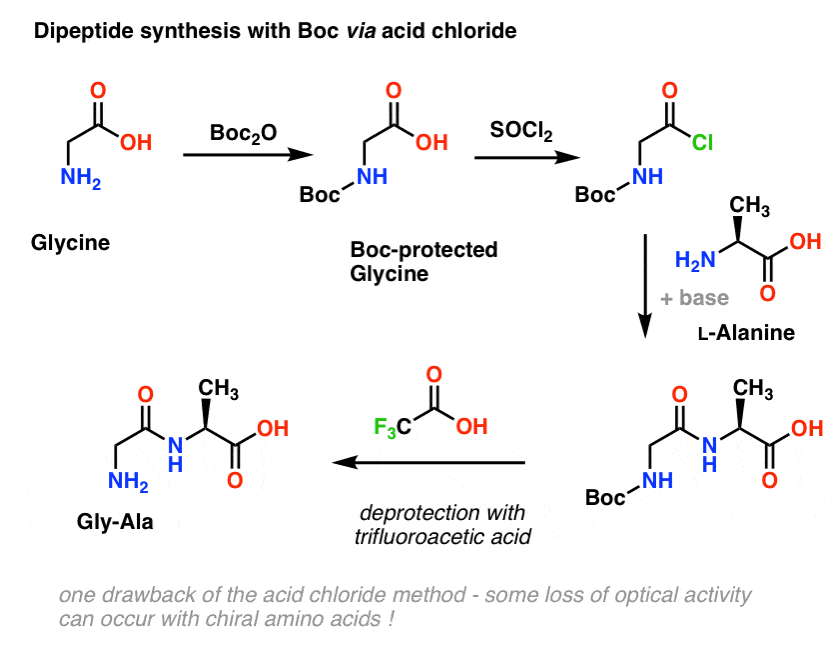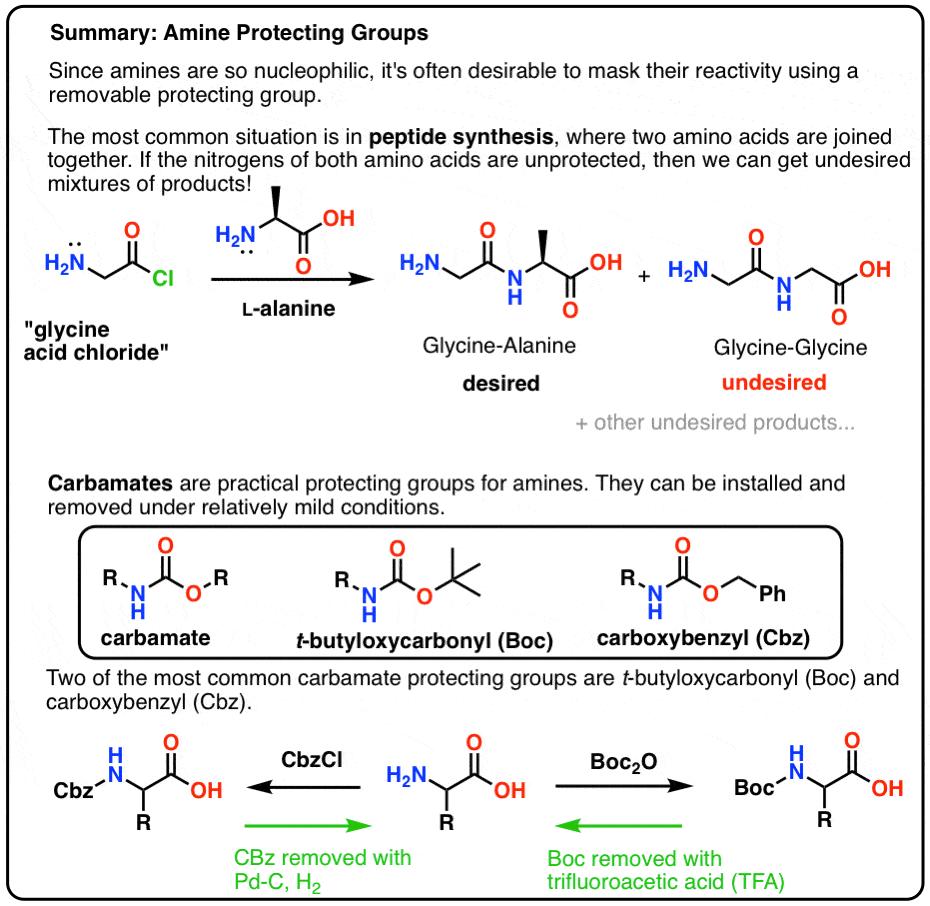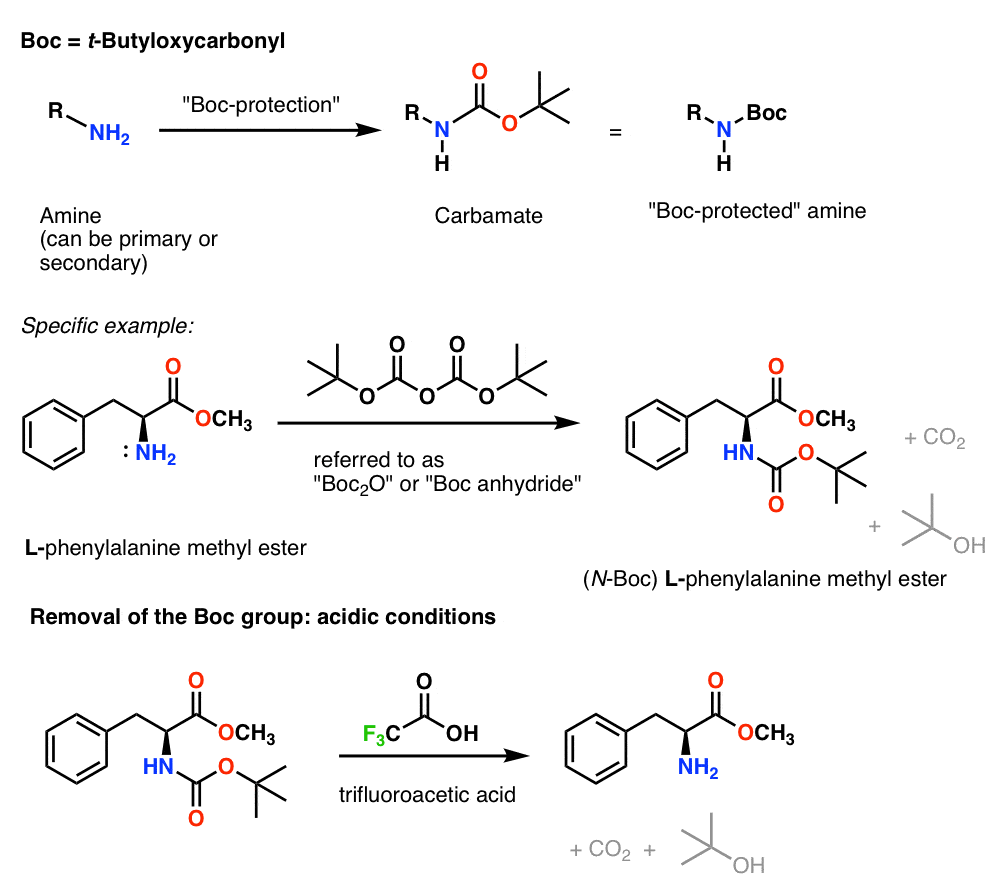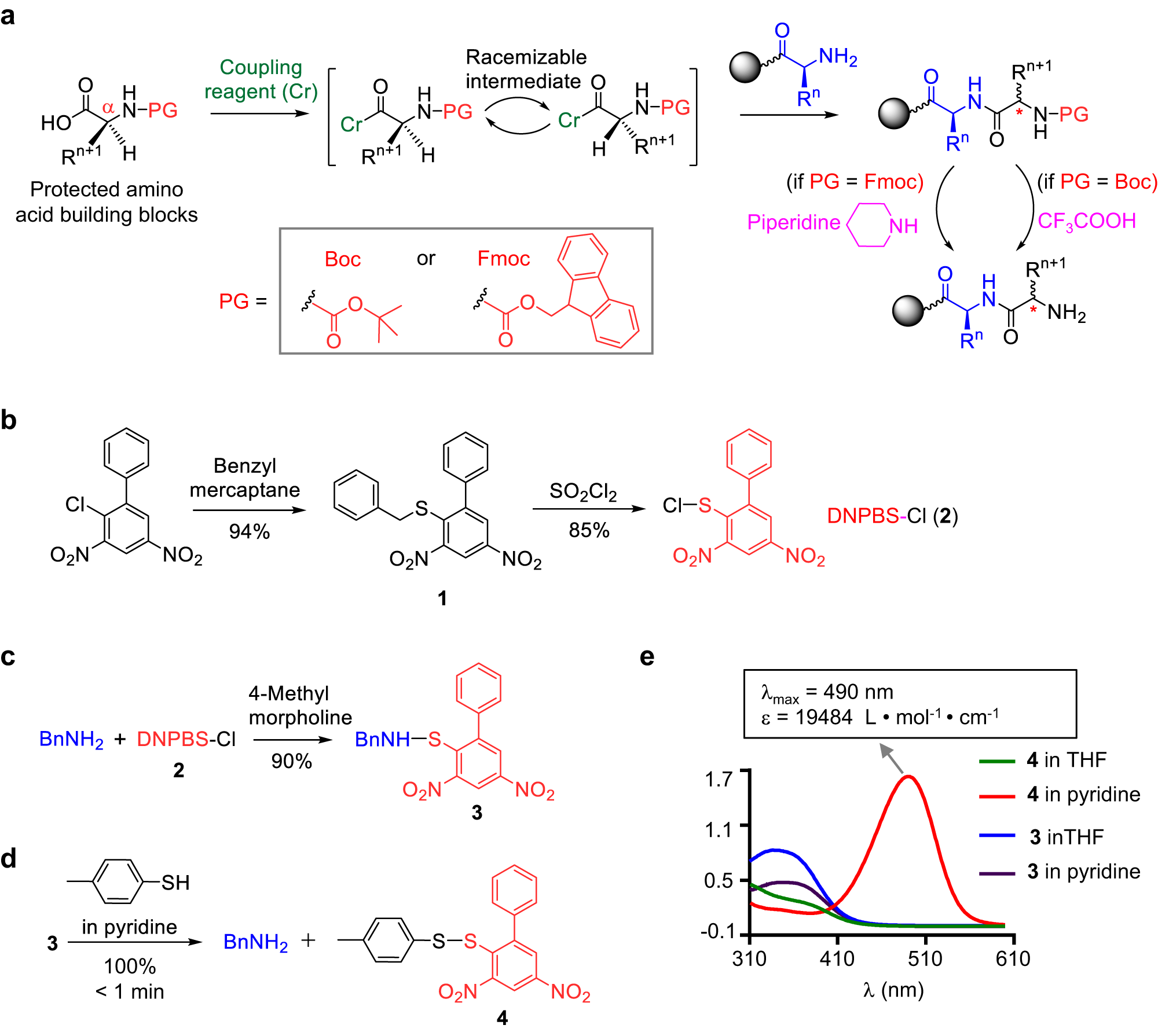
Suppression of alpha-carbon racemization in peptide synthesis based on a thiol-labile amino protecting group | Nature Communications
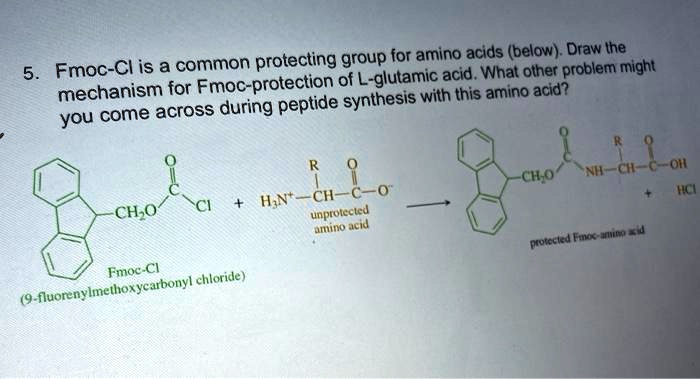
SOLVED: Fmoc-Cl is a common protecting group for amino acids. What other problem might arise with this amino acid during peptide synthesis?

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications

Selective Deprotection of N-Boc-Protected tert-Butyl Ester Amino Acids by the CeCl3*7H20-NaI System in Acetonitrile

Molecules | Free Full-Text | Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium(II) Iodide as a Protective Agent